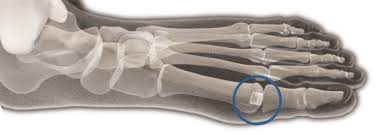
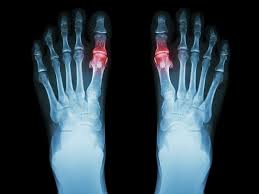
WHO SHOULD NOT RECEIVE THE CARTIVA® SCI (CONTRAINDICATIONS)?
Tell your doctor if you think you have an infection in your foot. An infection makes it risky to have the Cartiva SCI.
Tell your doctor if you think you have ever had any allergy to or reacted to any plastic or an implant.
The Cartiva SCI is made from a plastic-like mixture (polyvinyl alcohol and saline). You could be allergic to it. An allergic reaction to the Cartiva SCI might mean you would need more surgery to remove it.
Tell your doctor if you have a form of arthritis called gout that also causes small lumps (tophi) to form under the skin around your joints. The Cartiva SCI might not work in your joint with this kind of arthritis.
Tell your doctor if you have any of the following conditions that can hurt implant support.
- Cancer
- Hip dislocation
- Brittle bones or bones that break easily
- You have taken a steroid medication in the past
- You had an organ transplant
- You have taken an immunosuppressant mediation in the past
- You have a history of any growths (tumors) in your bones
These conditions might lead to changes in your bone that might make the Cartiva SCI device unable to work properly. You should speak to your doctor to determine if the above conditions apply to you, or if other conditions may make the Cartiva SCI not right for you.